Carbohydrate Digestion and Absorption
Learning Objectives
Describe digestion of carbohydrates starting in the oral cavity
Apply knowledge of carbohydrate structure to carbohydrate- specific digestive enzymatic properties
Compare the enzymatic breakdown of \(\alpha\)-1,4 vs. \(\alpha\)-1,6 glycosidic linkages.
Discuss the role of intestinal brush border enzymes in monosaccharide liberation.
Understand absorption of monosaccharides via glucose transporters
Explain transportation mechanisms of monosaccharides to target organs
Define glycemic index and its relation to chronic disease
Explain the role of insulin in maintaining blood glucose levels
Explain the history and etiology of lactase persistence
Carbohydrate Digestion
The most common classes of carbohydrates that are in food sources are di- and polysaccharides. The human body must break down these carbohydrates to their respective monosaccharide units because this is the form that can be absorbed across the enterocytes at the small intestine. The enzymes that break down carbohydrates to monosaccharides are collectively called glycosidases.
Oral Cavity and Stomach
The first exposure of a carbohydrate to a glycosidase occurs in the mouth. The salivary glands produce the enzyme alpha amylase, which is released into the mouth as part of saliva. Alpha amylase targets the oxygen bridges as part of alpha 1,4 glycosidic bonds between glucose unitsThis will be a recurring theme in this lecture, that particular enzymes can only digest specific glycosidic bonds. Keep in mind the limited arsenal of enzymes we have, relative to the very large number of potential glycosidic bonds. Its optimal activity occurs in pH around 6.5–7 As food is mixed with saliva and chewed, the carbohydrates are chemically and mechanically broken down. This is a relatively short period of digestion that produces oligo- and shorter polysaccharides and very few monosaccharides. As food travels from the mouth to the stomach, the salivary alpha amylase is still present. Once the bolus reaches the stomach there is a very limited period of digestion because the acidic environment alpha amylase activitySalivary alpha amylase is inactivated at pH less than (more acidic) than 4, so is not active beyond the upper stomach. Think of it as the first leg of a relay, it gets things started but passes the baton to pancreatic alpha amylase in the small intestine..
Taste of sugar. We have receptors for each of the five tastes; sweet, sour, bitter, salty and umamiThough the map you may be visualizing of localized taste receptors on the surface of the tongue is fake news. The sweet receptors can bind to several mono- and di-saccharides including fructose, sucrose and glucose but also other non-caloric compounds such as saccharin, sucralose and aspartame. Interestingly the taste receptorsEncoded by the genes __ELEM_140269689954624__ and __ELEM_140269689955200__ are also expressed throughout the gastrointestinal system suggesting that sugar sensing occurs at more locations than just the tongueT1R2/T1R3 activation in enteroendocrine cells influences GLP1 release __ELEM_140269689898624__.. For more about this emerging area check out this review by Janssen and Depoortere (2013)
Hormonal Modulation and Gastric Emptying. The rate at which chyme leaves the stomach and enters the small intestine—called gastric emptying—has a profound effect on postprandial blood glucose levels. Faster emptying delivers carbohydrates more rapidly to the absorptive surface of the small intestine, leading to a sharper rise in blood glucose. Conversely, slower emptying blunts the glycemic response.
Several hormones secreted in response to nutrients modulate this process:
Gastric Inhibitory Peptide (GIP) is secreted by K cells in the proximal small intestine. It enhances insulin secretion and slows gastric emptying modestly.
Glucagon-Like Peptide-1 (GLP-1) is secreted by L cells in the ileum and colon in response to carbohydrates and fats. It strongly delays gastric emptying and promotes satiety.
Cholecystokinin (CCK) is released in response to lipids and proteins and also contributes to delayed gastric motility.
These hormones are part of the incretin effect—the amplification of insulin secretion when glucose is ingested orally vs intravenously. This effect is partly due to the slowing of gastric emptying, which spreads glucose absorption over a longer time window.GLP-1 analogs (like semaglutide) are used clinically in type 2 diabetes and obesity to improve glycemic control and reduce appetite by mimicking this hormonal regulation.
Dietary fiber—especially soluble fiber—acts synergistically by increasing chyme viscosity, delaying stomach emptying, and slowing intestinal glucose absorption.
Small Intestine
When the bolus reaches the small intestine, the salivary alpha amylase is activated again because of the increase in pH. In addition to the salivary alpha amylase, the pancreas releases pancreatic juice that contains pancreatic alpha amylase. Amylose is broken down to maltriose (trisaccharide) and further more to maltose. Alpha amylase can work on the linear chain of \(\alpha\)-1,4 bonds of amylopectin but cannot hydrolyze the branching points that are \(\alpha\)-1,6 bondsWe do not have enzymes that can digest __ELEM_140269689899984__-1,4-bonds between glucoses such as those in cellulose, making it a fiber.. The product of amylopectin digestion from alpha amylase is a disaccharide called isomaltose.
The brush border of the small intestine houses glycosidases that help with the completion of carbohydrate digestion. For the purposes of our discussion we will focus on four of these enzymes. Maltase is specific to the \(\alpha\) 1,4 bond in maltose and maltriose. Isomaltase is specific to the \(\alpha\)-1,6 (i.e., former branching points) of isomaltoseCongenital sucrase-isomaltase deficiency (CSID) is a rare genetic disorder that leads to severe carbohydrate intolerance.Patients with CSID cannot digest sucrose or starch properly and often present with chronic diarrhea and failure to thrive in infancy.Treatment often involves a sucrose-free, starch-restricted diet, and in some cases oral enzyme replacement therapy (__ELEM_140269690050624__, sacrosidase). Consider why CSID would impair digestion of starch __ELEM_140269690053376__ sucrose.. Then considering the other two disaccharides abundant in our diet, lactose and sucrose, lactase and sucrase are specific for the \(\beta\)-1,4 and \(\alpha\)-1, \(\beta\)-2 bonds of lactose and sucrose, respectivelySucrase/isomaltase is actually one bifunctional enzyme which has two different domains that can catalyze each of these separate reactions..
Monosaccharide Absorption and Transportation
Monosaccharides, like glucose, are impermeable to cellular membranes and require carrier-mediated systems to cross the apical and basolateral membranes of the enterocyte in order to reach circulation. There are two transport systems important for absorption across the enterocyte. The first is an active carrier-mediated transport system called the sodium-glucose transport 1 (SGLT1). To work, this system requires energy through a sodium-potassium ATPase pump (Figure \(\ref{fig:glucose-intestine}\)). The other is a series of carrier-mediated transporters called Glucose Transporters (GLUTs) that work by facilitated diffusion. There are 14 GLUT isoforms that have been identified, but the best characterized are GLUTs 1, 2, 3, 4 and 5. Each GLUT has specific tissue distribution throughout the body, for example GLUT 2 and 5 are the isoforms located in the small intestine (see Table \(\ref{tab:glut-transporters}\)).
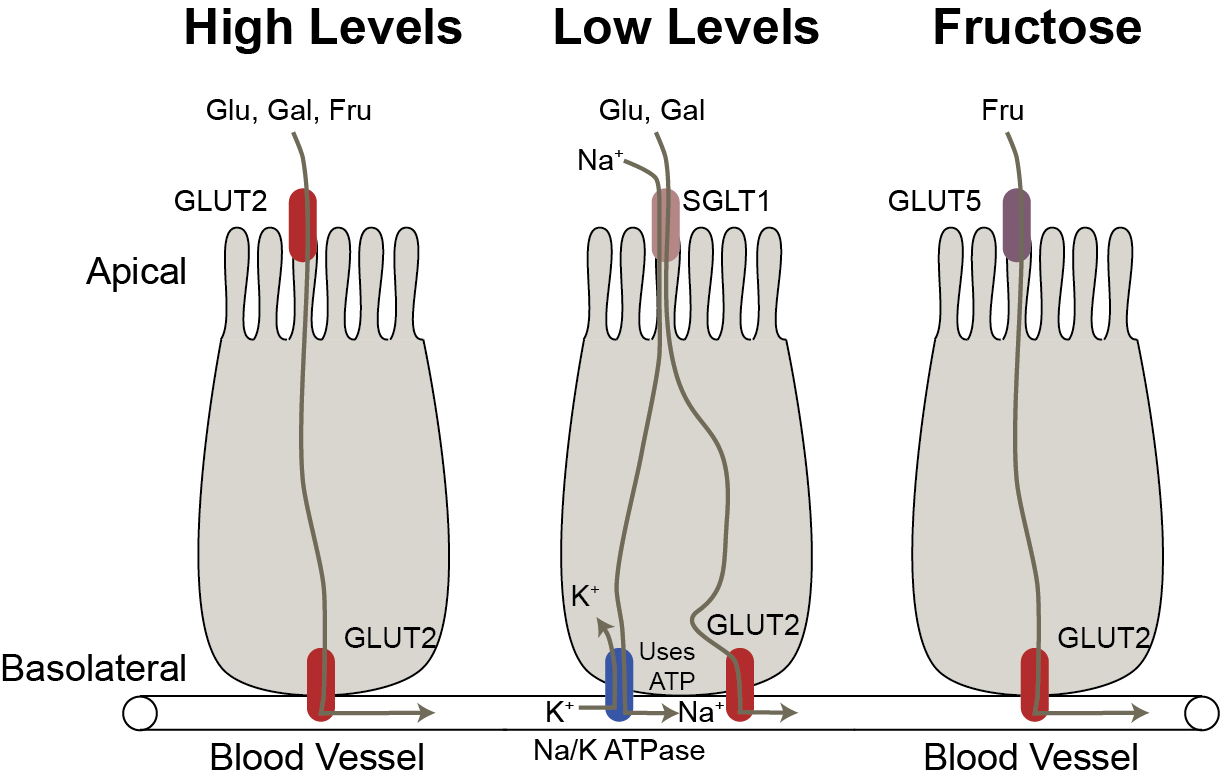
Glucose and Galactose
When glucose and galactose are present in lower concentrations in the lumen in comparison to the enterocyte, they require the SGLT1 transmembrane transport protein to cross the apical membrane. This transport system relies on the high sodium concentration in the lumen.
SGLT1 binds to glucose or galactose and to two sodium ions. Once sodium is bound, this allows for passive diffusion through the apical membrane into the enterocyte. Glucose and galactose are then released and carried through the basolateral membrane via GLUT2. To keep the lumen sodium concentrations high and enterocyte sodium concentrations low, the Na/K ATPase pump (energy required) pumps sodium out of the cell and potassium in, thus making this an active carrier-mediated transport system (Figure \(\ref{fig:glucose-intestine}\)).
Fructose
Fructose is transported by GLUT5 across the apical membrane and by GLUT 2 across the basolateral membrane. Recent work has shown that this system is easily saturable (Jang et al. 2018). At low levels of fructose, it is often converted to glucose and exported to the blood but at higher levels it can enter the circulation as fructose or stay in the gastrointestinal tract resulting in fermentation by the gut microbiota. That in turn can become acetate and result in hepatic lipogenesis (Zhao et al. 2020).
Glucose, Fructose and Galactose — under conditions of high lumen concentrationsFollowing high carbohydrate intake, SGLT1 becomes saturated so GLUT2 is translocated to the apical membrane to assist with transportation across the membrane via passive diffusion (Kellett et al. 2008). Under this mechanism the three monosaccharides will still cross the basolateral membrane via GLUT2. In addition to SGLT1 saturation, the activation of the “sweet taste” receptors in the enteroendocrine cells of the digestive tract have been shown to play a role in the translocation of GLUT2 to the apical membrane. This is triggered by monosaccharides as well as sugar substitutes (Stearns et al. 2010). The small intestine is sensitive to insulin, which is released from the pancreas following glucose absorption. The insulin response results in the internalization of GLUT2 (translocation away from the apical membrane) thus decreasing the absorption rate of glucose. Lastly, mRNA expression of GLUT2 is increased after consumption of a high carbohydrate meal (Miyamoto et al. 1993). This is not unique to GLUT2, but has also been found for GLUT5 and SGLT1. Thus, there are multiple levels of transporter regulation at the small intestine that influence monosaccharide absorption.
Systemic Carbohydrate Transport
Once the monosaccharides cross the basolateral membrane, they are small enough molecules that they are able to enter the capillary system eventually entering the hepatic portal vein leading to the liverhepatic = liver. Galactose and fructose will undergo metabolism in the liver and sm all intestine to be converted into glucose and its derivatives. Glucose can be stored as glycogen, oxidized for energy, broken down for fatty acid or amino acid synthesis or reenter circulation to tissues, all dependent on the body’s energy status.
Transportation across cell membranes outside of the intestine requires glucose transporters. As stated earlier, the glucose transporters are tissue specific thus the isoform present is dependent upon the tissue type (Table \(\ref{tab:glut-transporters}\)). Glucose requires insulin signaling to be taken up by GLUT4 at skeletal muscle and adipose tissue.
| Isoform | Expression | Substrates | |
|---|---|---|---|
| GLUT1 | CNS, Placenta | Glu, Gal | |
| GLUT2 | Liver, Pancreas, Small Intestine | Glu, Gal, Fru | |
| GLUT4 | Adipose, Muscle | Glu | |
| GLUT5 | Small Intestine | Fru |
Insulin Resistance and Diabetes
GLUT4 is a key glucose transporter found on muscle and adipose tissue. This transporter is unique because to function properly it is dependent on proper insulin signaling. Under normal conditions, pancreatic beta-cells respond to an increase in blood glucose levels by secreting insulin into the bloodstream. Insulin receptors are located on skeletal muscle and adipose tissue that bind insulin under these conditions. Once insulin is bound this sends a phosphatidylinositol-3-kinase signal cascade within the cell that releases stored GLUT4 from golgi apparatus in the form of a GLUT4 storage vesicle (GSV). The GSV carries GLUT4 to the cell membrane, releases it and allows for passive diffusion of glucose into the cell.
Insulin insensitivity is a condition that is associated with obesity, Type 2 Diabetes and other chronic conditions (DeFronzo 2010). It occurs when the muscle and adipose tissue cells become unresponsive to this insulin signal for cellular glucose uptake resulting in elevated blood glucose levels. Because glucose remains in the blood, the pancreas secretes additional insulin to continue to work on getting glucose into cells and also stimulates gluconeogenesis (biosynthesis of glucose by the liver) because it thinks the cells are deprived of glucose. When this is a continuous occurrence, the pancreatic beta-cells become overworked and stop producing sufficient insulin resulting in long-term hyperglycemia leading to Type 2 Diabetes, heart diseaseand other vascular disease. There are several mechanisms that have been hypothesized as playing a role in the mechanism of a cell becoming unresponsive to the insulin signal such as inflammation, genetics and epigenetics. One studied mechanism is lipotoxicity in which excess fat buildup within the body results in fat residing on or within cellular space. This can result in disruption of the insulin pathway by cellular damage and inflammation.
Glycemic Index
For individuals who suffer from hyperglycemia, diet can play a key role for controlling their blood level of glucose throughout the day. Carbohydrate-containing food have been categorized by a glycemic index. This index is an indication of the absorption rate of monosaccharides from the small intestine after consuming carbohydrate-containing food (Atkinson et al. 2008; Dodd et al. 2011).
The index ranges from 1–100 where 100 is the reference of the fastest absorption potential and often symbolized by white bread. As a rule of thumb, foods higher in fiber and lower in fat have a lower glycemic index and more processed foods (decreased fiber content) have a higher glycemic index (Table \(\ref{tab:glycemic index}\)). The glycemic index is a tool that can be used to help educate individuals on proper food choices to help control flux of carbohydrate absorption and to help maintain a steady level of blood glucose when consuming food and throughout the day. The glycemic index is not a perfect tool, however, as it does not take into account the amount of carbohydrate in a food. For example, watermelon has a high glycemic index but low carbohydrate content, so it is not a significant source of glucose. The glycemic load is a more accurate measure of the impact of a food on blood glucose levels as it takes into account both the glycemic index and the amount of carbohydrate in a serving of food (Atkinson et al. 2008).
Dietary fiber, particularly viscous soluble fiber, slows the rate of carbohydrate absorption. It forms a gel-like matrix in the lumen of the small intestine, increasing the viscosity of chyme and delaying gastric emptying. This matrix creates a physical barrier that:A meta-analysis of observational cohort studies shows that for every 10g/day increase in total fiber intake, risk of type 2 diabetes decreased by 9% __ELEM_140269689912224__.__ELEM_140269689912384____ELEM_140269689912464____ELEM_140269689912544__.
Reduces enzyme-substrate contact (e.g., \(\alpha\)-amylase can’t reach starch as easily).
Slows the diffusion of monosaccharides to the enterocyte surface through increased viscosity.
Delays glucose transporter engagement by reducing the movement of monosaccharides to the enterocyte.
Lactase Non-persistence
Lactose intolerance is a common term used to describe the overarching symptom of the condition of lactase non-persistence. This condition results from an enzyme deficiency of the digestive enzyme lactase, described above. In mammals, the lactase geneThe gene symbol is __ELEM_140269688062080__ is highly active in the lactation life stage (i.e., infancy) when the sole food intake is from the mother’s lactose-containing breast milk. Following the introduction to other food products, and consequently, exposure to foods containing carbohydrates other than lactose, the production of lactase rapidly declines, as there is no significant need for its activity.
Because of the innovation of domesticating milk-producing animals, humans are a unique mammal because this allows us the opportunity to continue to drink milk and eat milk-based food products beyond the lactation period (Itan et al. 2009). Over time some humans developed a genetic polymorphism in the lactase gene resulting in sufficient production of lactase throughout adulthood when there is continuous exposure to lactose-containing foods. Because milk-producing agriculture is a recent event in regard to evolution this polymorphism is thought to arise from a high frequency haplotype due to the benefit of ingesting lactose in cultures that raise milk-producing animals (Harvey et al. 1998; Itan et al. 2009). Individuals lacking this polymorphism will have a decline in lactase production and will start to have symptoms of lactose intolerance in early childhood.
Specific symptoms include bloating, cramping and diarrhea. Because the disaccharide lactose will not be properly digested for absorption, it will travel to the large intestine where it can absorb and retain water within the large intestine causing bloating and potentially diarrhea and dehydration. Of note, it has been evidenced that individuals who are lactase persistence but do not expose themselves to milk-containing products will have a steady decline of lactase production over time and may also exhibit symptoms of lactose intolerance when reintroducing milk-products (Gerbault et al. 2011).